Matter (High School Science)
THE DIFFERENT BUILDING BLOCKS OF MATTER
Matter may be defined as: The “material” the universe is made up of; and atoms are the building blocks of matter.
Atoms are the smallest particles of an element that still have the element’s properties.
Elements, in turn, are pure substances that make up all matter.
Atoms, however, are not the smallest particles of matter.
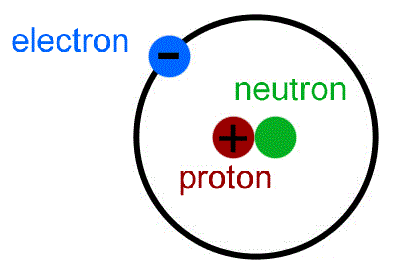
Atoms are made up of even smaller, subatomic particles called protons, neutrons and electrons.
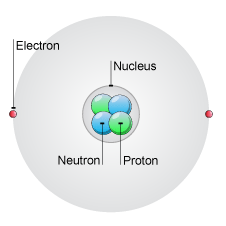
At the centre of every atom is a nucleus containing protons and neutrons.
All atoms of a given element are identical in that they have the same number of protons. This number is used to arrange the elements in the Periodic Table, beginning with hydrogen, which has just one proton.
Electrons are contained in shells around the nucleus. The total number of electrons is always the same as the number of protons in the nucleus.
Protons and electrons have an electrical charge. This electrical charge is the same size for both, but protons are positive and electrons are negative.
Neutrons have no electrical charge; they are neutral.
What are ions?
Ions are electrically charged particles which are formed when atoms gain or lose electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.
HYDROGEN, OXYGEN, CHLORINE ATOM DIAGRAMS
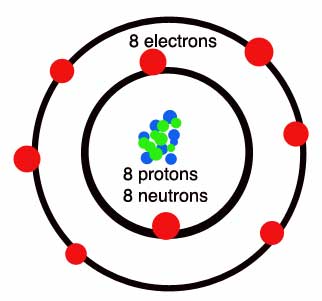
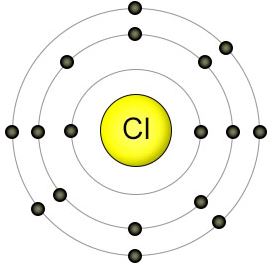
DIFFERENTIATE ELEMENTS, COMPOUNDS AND MIXTURES
Elements
All matter is made from atoms. Atoms are very, very small. A molecule is formed when atoms join together by chemical bonds.
There are over a hundred different types of atom, called elements. The atoms of a particular element are identical to each other. They cannot be changed chemically into any different element. For example a piece of pure sulfur consists only of sulfur atoms. No chemical process can break it down into a different substance.
Compounds
Compounds are substances that contain atoms of at least two elements chemically combined. For example: magnesium oxide consists of magnesium atoms and oxygen atoms chemically bonded together.
Mixtures
Many substances exist as mixtures of other pure substances. The properties of a mixture are always the same as the properties of the substances which make up the mixture.
- Air is a mixture of gases
- Sea water is a mixture of substances dissolved in water
- Crude oil is a mixture of hydrocarbons
| Examples of compounds and elements | ||
| hydrogen | hydrogen | an element |
| sodium chloride | sodium and chlorine | a compound |
| chlorine | chlorine | an element |
| carbon dioxide | carbon and oxygen | a compound |
FORMATION OF COVALENT AND IONIC COMPOUNDS
Covalent bonds
A covalent bond is formed between non-metal atoms, which combine together by sharing electrons.
Covalent compounds have no free electrons and no ions so they don’t conduct electricity.
Non metals combine together by sharing electrons. The shared pair of electrons holds the two atoms together. It’s called a covalent bond. The group of atoms bonded together in this way is called a molecule.
The types and numbers of atoms in a molecule are shown in its formula.
| Name | Structure | Model |
| Hydrogen (H2) | ||
| Water (H2O) | ||
| Ammonia (NH3) |  |
 |
| Methane (CH4) |  |
 |
Covalent compounds are usually gases or liquids with low melting points or boiling points and they don’t conduct electricity.
FORMATION OF SODIUM CHLORIDE AND AMMONIA
Sodium chloride is a compound formed from the ionic bonding of sodium and chloride. The result is a salt that is very important biologically and commercially. This article discusses sodium chloride, its properties, and its uses.
Salts are inorganic compounds, which means they do not contain carbon and hydrogen together in one molecule. Salts form when a positively-charged atom, called a cation, attracts a negatively charged atom, known as an anion.
This positive to negative attraction is known as an ionic bond and is key in maintaining the chemical structure of salts. One of the most important salts in nature and biological systems is sodium chloride. Let’s look at sodium chloride and discuss what makes it such a valuable salt in nature.
Sodium chloride is formed when sodium atoms interact with chlorine atoms. When this occurs, sodium will donate an electron (which is a negatively-charged particle) to chlorine. This makes sodium slightly positive and chlorine slightly negative.
Opposite charges attract, right? So then, sodium ions will attract chloride ions and form an ionic bond. By the way, chloride is the term used to designate the anion form of chlorine. The result is a crystallized salt that has properties that are different from the two parent elements (sodium and chlorine). The chemical formula for sodium chloride is NaCl, which means that for every sodium atom present, there is exactly one chloride atom.
Sodium chloride has a molar mass of 58.44 grams per mole. It appears as a solid, clear crystal with little or no odor. As a salt, sodium chloride dissolves well in water and the ions in the crystals will separate when in solution. Have you ever added salt to a pot of boiling water, maybe to make pasta? You can see how instantly the little crystals dissolve, or break apart in the water.
Sodium chloride molecules can also stack on top of each other in a structure known as a lattice and the solid crystals of sodium chloride will contain this lattice-type arrangement.
FORMATION OF AMMONIA (NH3)
Nitrogen gas (N2) and hydrogen gas (H2) can be made to combine to form NH3, or ammonia gas. As we proceed, look for the answers to these questions: Is the reaction exothermic? How many kilograms of hydrogen are needed to produce one kilogram of ammonia?
The reaction is N2 + 3H2 → 2NH3
This equation says that one mole of N2 requires three moles of H2 for a complete reaction, and this would then yield two moles of NH3. Note that we can also say that one molecule of N2 reacts with three molecules of H2 to yield two molecules of ammonia (NH3). But the table gives energies in units of kcal/mole, which is why it is easier to work with moles. The energy involved in the reaction involving just one or two molecules of ammonia is too small.
The following contains the variety two ways in which you can write the reaction that forms ammonia.
Nitrogen + Hydrogen → Ammonia
N2 + 3H2 → 2NH3
Matter (High School Science) Read More »